Aldehyde reduction by the numbers
07 December 2024 - Research update 00003
Planning an aldehyde reduction? We have the numbers! Currently in stock 4000 entries. No surprises with respect to the top reducing agents, they are all borohydrides with sodium borohydride holding on to a 88% market share. In third place we have lithium aluminium hydride with just 3%. The ratio of reducing agent to reaction may be a surprise because a ratio of 1:1 accounts for just 44% of all entries. Ratio's go up to 1:8 with at the extremes 100-fold to 1000-fold excesses, we may assume human-error at the weighing station, a rush-job , confusing milligrams with grams or simple typo's in the write-up?
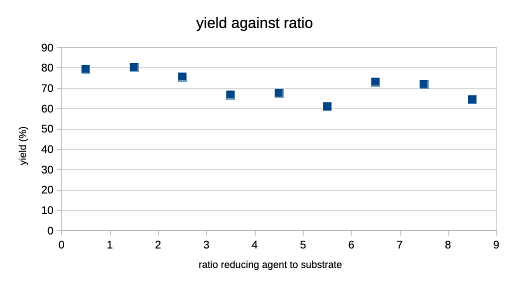
The ratio's do not seem to impact the reported yield much, which slide from 80% to 60-70% going up. Reaction temperature is not much of an issue, either a cool 0 or an ambient 25. Solvent then? The solvent top three is methanol, THF and ethanol.
In any event, the reported reaction times vary from 1 minute all the way to 420 minutes, then nothing happens until minute 840 when everybody is back in the office the next day to finish up the reaction (chemistry do have to sleep).
The different reducing agents still have to rationalized. According to Jerry March LiAlH4 is fast but not selective. NaBH4 is slower but leaves these groups alone: nitro, aliphatic chlorine, ester and nitrile. Thanks to RdKit is is possible to query reaction SMILES not just for reaction type but at the same time also check functional group tolerance: if a functional group is present in both the reactant and the product than the reaction type has tolerance for this functional group. And do the reactions in the Chemical Reaction Database (CRD) behave as predicted? The answer is yes: the aluminium compound has not a single reaction with either a nitro, chlorine, ester or nitrile group whereas boron reducing agent have plenty (18% chlorine, 17% ester, 5% nitrile, 3.4% nitro). The picture for the number 2 reducing agent, sodium triacetoxyborohydride is less clear. This compound is more typically associated with reductive aminations and some of them weaseled their way into this dataset.
It is also mentioned in the literature (Periasamy, 2000) that sodium triacetoxyborohydride is chemoselective for aldehydes with respect to ketones but even though 4 such examples (aldehyde reduction in presence of a ketone group) can be fished out of the data for the acetoxyborohydride, the regular borohydride group has 12, half of them with the ketone group left intact, so even with a dataset of 4000 it is not possible to really draw conclusions. Other reducing agents such as (green) ammonia borane (Shi, 2012) or ter-butylamine borane (Burkhardt, 2006) touted in the literature have not made it into the mindset of the average organic chemist.
