Novel aromatic deamination
18 April 2009 - News
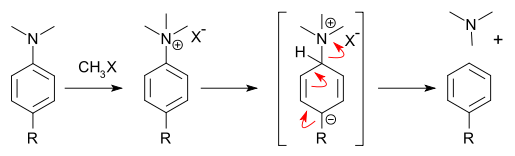
The David MacMillan group recently came up with a way to remove an amino group from an arene. In this way the amine can direct an electrophilic aromatic substitution in a specific way and later on be discarded, very much like a sulfonate group (deactivating rather than activating). The strategy employed is methylation of the amine, followed by a Birch reduction and a kind of vinylogous Hofmann elimination (DOI):
:
The novel procedure was demonstrated with a synthesis of Tolterodine:
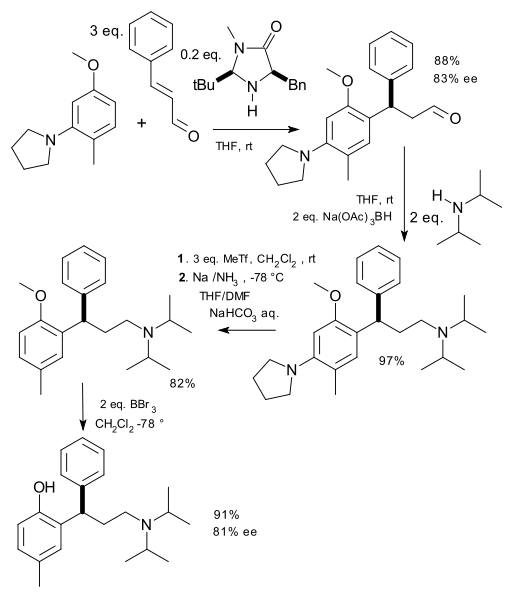
as a sequence of an asymmetric Friedel-Crafts reaction using cinnamaldehyde, an pyrrolidinoanisole and a McMillan catalyst, followed by a reductive amination (diisopropylamine, sodium triacetoxyborohydride), removal of the pyrrolidinone group by methyl triflate and sodium/ammonia treatment, followed by ether deprotection (boron tribromide) to the phenol.