Platencin update
26 April 2009 - total synthesis
You have been warned (previous post here), this is platencin total synthesis number 10 (Varseev & Maier 2009 DOI).
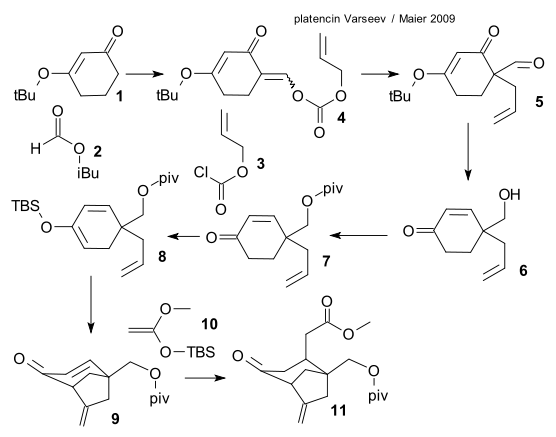
Starting material is 3-isobutoxycyclohex-2-en-1-one 1, formylation with isobutyl formate 2 is followed by nucleophilic substitution to allyl chloroformate 3 forming an E/Z mixture of allyl carbonate 4. A Carroll rearrangement introduces the quaternary carbon atom in 5 (step also performed as an asymmetric synthesis), an hydride reduction to alcohol 6 is followed by protective group addition to pivaloyl ester 7. Conversion to silyl enol ether 8 is followed by cyclization (oxygen, palladium acetate) to exocyclic alkene 9. Addition of Ketene silyl acetal 10 forming 11 is a Mukaiyama-Michael Addition.
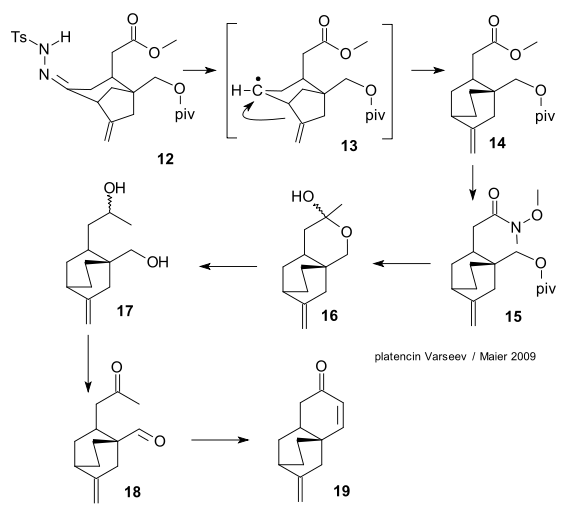
The ketone 11 is converted to hydrazone 12. This compound reacts with NaCNBH3 and ZnCl2 to free radical intermediate 13 which rearranges the 3.2.1 platensimycin core to the 2.2.2 platencin core in 14. Conversion of the ester group in 14 to a ketone takes place through Weinreb amide 15 and MeLi , the intermediate 16 is a hemiacetal. The final steps are reduction (LiAlH4) to diol 17, Swern oxidation to aldehyde 18 and a ring-closing aldol condensation to the platencin core 19.