Amide Umpolung aller Werte
30 June 2010 - Organic chemistry
 Amides are typically made from a nitrogen nucleophile (amine) and an activated electrophilic carbonyl compound (for example acid chloride) in the Schotten-Baumann reaction. This functional group is relevant to biochemistry as peptides and chemists are always on the lookout for new synthetic methods. A novel approach by Shen et al. reverses the parts played by nucleophile and electrophile in what is known in organic chemistry as umpolung (DOI).
Amides are typically made from a nitrogen nucleophile (amine) and an activated electrophilic carbonyl compound (for example acid chloride) in the Schotten-Baumann reaction. This functional group is relevant to biochemistry as peptides and chemists are always on the lookout for new synthetic methods. A novel approach by Shen et al. reverses the parts played by nucleophile and electrophile in what is known in organic chemistry as umpolung (DOI).
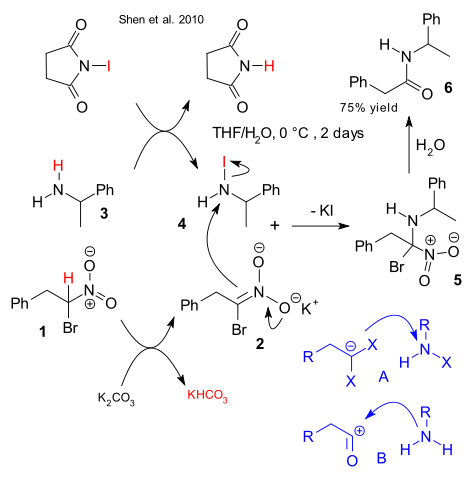
In the new scheme alpha bromo nitroalkane 1 forms a nucleophilic synthon after deprotonation by potassium carbonate to nitronate salt 2. At the same time 1-phenylethylamine 3 exchanges a proton for an iodine atom with NIS to N-iodiamine 4. The nucleophilic attack by the nitronate is similar to what can be seen in electrophilic amination by an enolate. Intermediate 5 after hydrolysis as in the Nef reaction gives amide 6.