Desiraju on hydrogen bonds
15 January 2011 - Chemical bonding
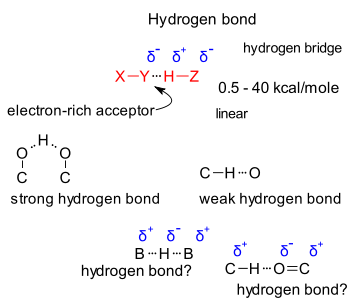 A hydrogen bond can depicted as an X-Y...H-Z interaction involving 0.5 to 40 kcal/moles of energy with the strongest hydrogen bond stronger than weakest covalent bond and the weakest H-bond in the range of a van der Waals bond. In an Angewandte essay Gautam R. Desiraju elaborates on the nature of the hydrogen bond and a proper definition with unusual clarity. (DOI). For those of you without an Angewandte subscription or with without patience to read all 8 pages, here is a brief capture. To start with just a few quotes:
A hydrogen bond can depicted as an X-Y...H-Z interaction involving 0.5 to 40 kcal/moles of energy with the strongest hydrogen bond stronger than weakest covalent bond and the weakest H-bond in the range of a van der Waals bond. In an Angewandte essay Gautam R. Desiraju elaborates on the nature of the hydrogen bond and a proper definition with unusual clarity. (DOI). For those of you without an Angewandte subscription or with without patience to read all 8 pages, here is a brief capture. To start with just a few quotes:
On definitions: The history of chemistry is strewn with names that were once hotly contested and disputed by various protagonists and why do chemists fight over names so much, good question.
On trivial names: a term is acceptable if the largest numbers of chemists are in a maximum degree of agreement about what it means. A trivial name is the victory of such a consensus
On chemical bonds: the word bond has an almost religious connotation for chemists (...) every chemist has his or her own idea as to what constitutes a bond
On the origin of life : did life on earth become water-based because the hydrogen bond was the only interaction of choice to facilitate life?
So what is the problem with these hydrogen bonds. Strong hydrogen bonds or hydrogen bridges found in O-H--O-R configurations pose no problem. Problems arise with weaker bonds, not always possible or difficult to detect it experimentally More disagreement: in the original Pauling definition and later IUPAC definition Y and Z are both electronegative as in R-O-H...O-R (electropositive hydrogen partners with two electronegative partners) while in an scenario with C-H...O=C-R the carbon atom is electronegative and the oxygen atom electronegative. So not a hydrogen bond then?
An elite team of IUPAC chemists of which Desiraju was part of have now decided on the future of the hydrogen bond. In a new definition the hydrogen bond is an attractive interaction between a hydrogen atom from a molecule or a molecular fragment X-H in which X is more electronegative than H, and an atom or a group of atoms in the same or a different molecule, in which there is evidence of bond formation. So far so good. The definition goes on with the evidence for hydrogen-bond formation may be experimental or theoretical, or ideally, a combination of both. Here Desiraju stresses that theory and experiment have equal status which may come as a surprise to those who believe in the ancient scientific principle that theories are validated by experiment. The new definition also stipulates that the actual bridge is formed by Y-H but if Desiraju has had his way the bridge would be formed by all 4 atoms in X-Y-H-Z.
The X to Y distance no longer needs to be smaller than the sum of the van der Waals radii of X and Y: the precision of X-ray analysis does not allow it.
Here is one more quote, this time on IUPAC meetings: the letters X, Y, and Z were selected after much discussion
