From hexane to benzene
12 February 2011 - Catalysis
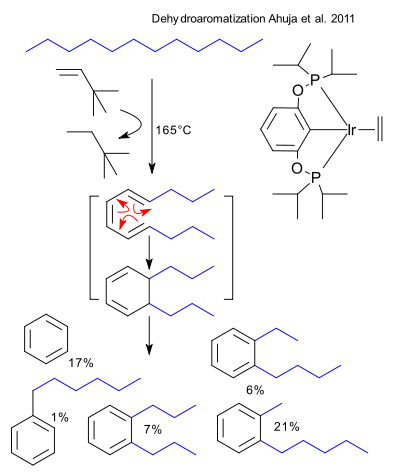 After reading this blog you will have learned how to synthesis benzene from n-hexane. Now you say hexane is at the most a solvent but not a reagent but then you have underestimated the power of catalysis. Ahuja et al. (DOI) claim to be first to use homogeneous catalysis with reasonable temperatures in the dehydroaromatization of linear alkanes. The technique is transfer hydrogenation with either t-butylethylene (TBE) or propene and the catalyst one based on iridium with a phosphine pincer ligand. Sure enough 165°C and 120 hours into the reaction benzene is formed at 44% conversion.
After reading this blog you will have learned how to synthesis benzene from n-hexane. Now you say hexane is at the most a solvent but not a reagent but then you have underestimated the power of catalysis. Ahuja et al. (DOI) claim to be first to use homogeneous catalysis with reasonable temperatures in the dehydroaromatization of linear alkanes. The technique is transfer hydrogenation with either t-butylethylene (TBE) or propene and the catalyst one based on iridium with a phosphine pincer ligand. Sure enough 165°C and 120 hours into the reaction benzene is formed at 44% conversion.
The real prize though is synthesis of alkylated arenes that are currently produced industrially from aromatics with Friedel-Crafts acylation and Clemmensen reduction. These aromatics are petroleum-based of which supply is dwindling whereas n-alkanes can be produced in the Fischer-Tropsch process from carbon monoxide and hydrogen gas.
The conversion of n-dodecane with 4 equivalents of TBE is believed to proceed by a triple dehydrogenation, followed by electrocyclization and another dehydrogenation. The yield of the actual objective - n-hexylbenzene - is meager (1%) but the total aromatics count is 52%. The authors also present a riddle: what is benzene (17%) doing in the mix? Somehow a carbon - carbon bond gets cleaved but n-hexylbenzene itself or hexylcyclohexane are resistant to cleavage when exposed to the reaction conditions and catalyst.
