NNNS Chemistry blog
Prevous: Table salt not as you know it
Next: Himmel, Krossing and Schnepf on dative bonds
Introducing the aromatic ene reaction
23 December 2013 - Orgo
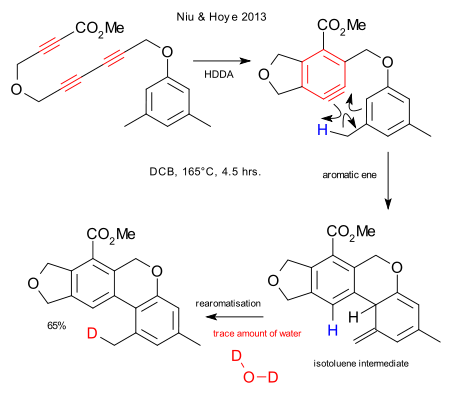 From the same people who brought you the hexadehydro-Diels-Alder reaction (HDDA), there is now the aromatic ene reaction (Niu & Hoye DOI). The HDDA reaction is a cyclotrimerization of a linear triyne to an aryne. This thermal reaction does not require any reagents and does not leave side-products so the aryne is produced very cleanly. In the subsequent aromatic ene reaction the ene is the methyl hydrogen and the enophile the alkyne. The potentially competing DA reaction is energetically less favourable. The isotoluene intermediate (known to chemistry but very obscure) quickly rearomatises. This step requires a trace amount of water (solvent dichlorobenzene). The isotoluene to toluene isomerization is symmetry forbidden but any proton donor can catalyse the reaction by proton shuttling. With deuterated water, deuterium is prominent on the reformed methyl group.
From the same people who brought you the hexadehydro-Diels-Alder reaction (HDDA), there is now the aromatic ene reaction (Niu & Hoye DOI). The HDDA reaction is a cyclotrimerization of a linear triyne to an aryne. This thermal reaction does not require any reagents and does not leave side-products so the aryne is produced very cleanly. In the subsequent aromatic ene reaction the ene is the methyl hydrogen and the enophile the alkyne. The potentially competing DA reaction is energetically less favourable. The isotoluene intermediate (known to chemistry but very obscure) quickly rearomatises. This step requires a trace amount of water (solvent dichlorobenzene). The isotoluene to toluene isomerization is symmetry forbidden but any proton donor can catalyse the reaction by proton shuttling. With deuterated water, deuterium is prominent on the reformed methyl group.
