Suzuki without metal
22 June 2014 - Orgo
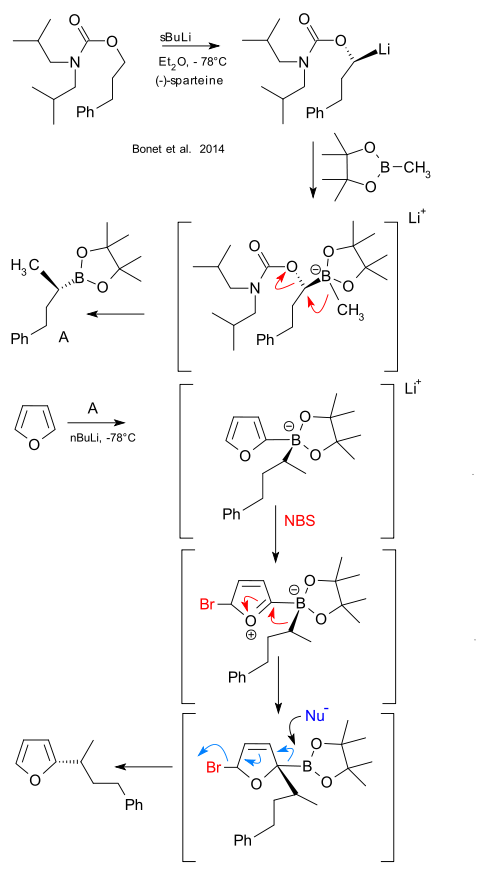 The Aggerwal group has recently described an alternative to the well-known Suzuki carbon - carbon coupling reaction that still utilises a boronic acid but does away with the platinum catalyst (Bonet et al. DOI). The novel method is applicable to aromatics such as furan, thiophene and electron-rich arenes. The metal is replaced by NBS acting as an electrophile. This novel research was triggered by a reinvestigation of some very old chemistry dating from the 1970's (DOI, DOI, DOI) with the key difference being that the Aggerwal boron shuttles are now chiral.
The Aggerwal group has recently described an alternative to the well-known Suzuki carbon - carbon coupling reaction that still utilises a boronic acid but does away with the platinum catalyst (Bonet et al. DOI). The novel method is applicable to aromatics such as furan, thiophene and electron-rich arenes. The metal is replaced by NBS acting as an electrophile. This novel research was triggered by a reinvestigation of some very old chemistry dating from the 1970's (DOI, DOI, DOI) with the key difference being that the Aggerwal boron shuttles are now chiral.
Chiral boron compound A (described in a 2007 publication) was synthesised from a carbamate of phenylpropanol that via its alkyllithium was stereoselectively methylated with pinacol methylboronate using sparteine. Compound A was then reacted with lithiumfuran to first give a boronate complex which was then brominated with NBS triggering a 1,2-shift (akin a Matteson homologization) followed by an elimination reaction with rearomatization to the chiral alkylated furan as final product.
Interestingly in the Aggerwal publication the final elimination is triggered by a nucleophile at least according to the published reaction scheme. Details on what the nucleophile might be however are lacking. In their Nature Chemistry editorial on the same topic Ho-Yan Sun and Dennis G. Hall must have struggled with the same problem and in their infographic the nucleophile was simply scrapped.
Update 23-06: the image did contain some errors. Corrected.
