Fructose to dimethylfuran
03 September 2010 - Biofuels
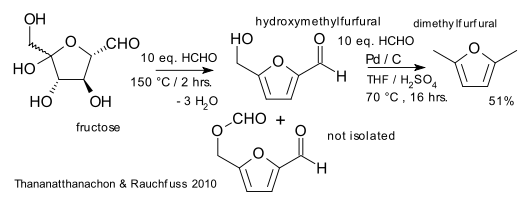 In a new proposal by Thananatthanachon and Rauchfuss (University of Illinois) the biofuel dimethylfuran (DMF) can from now on be made from fructose in a single reactor with formic acid and palladium on carbon (DOI). Fructose can be obtained from glucose by isomerization and ultimately from cellulose. DMF is an attractive biofuel because of its high boiling point (around 100°C), its high energy density and its high research octane number (little below ethanol).
In a new proposal by Thananatthanachon and Rauchfuss (University of Illinois) the biofuel dimethylfuran (DMF) can from now on be made from fructose in a single reactor with formic acid and palladium on carbon (DOI). Fructose can be obtained from glucose by isomerization and ultimately from cellulose. DMF is an attractive biofuel because of its high boiling point (around 100°C), its high energy density and its high research octane number (little below ethanol).
The purpose of formic acid in the new procedure is threefold: proton source for fructose dehydration (acid catalyst) to the hydroxymethylfurfural (HMF) intermediate, a hydrogen source for the hydrogenation step (to the bis(hydroxymethyl)furan intermediate) and as a deoxygenation agent.
